Claiborne Medical Center receives COVID-19 vaccine
Published 10:00 am Tuesday, December 22, 2020
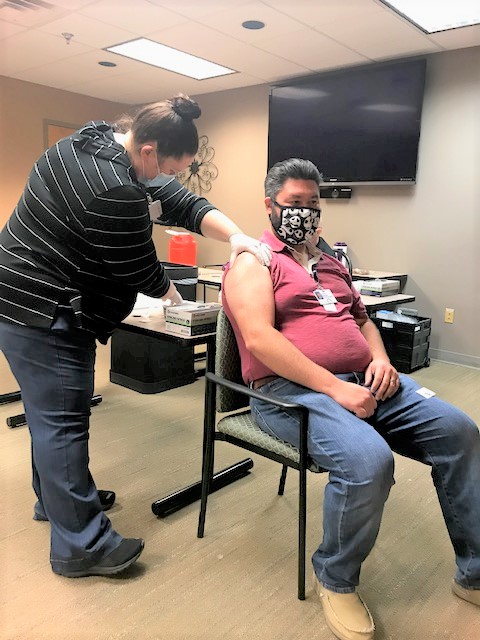
- Roger Mason, who is the manager of the Claiborne Medical Center Emergency Department, receives the first shot of the Pfizer/BioNTech vaccine to combat COVID-19. (courtesy photo)
|
Getting your Trinity Audio player ready...
|
The first shipment of the Pfizer/BioNTech (mRNA) COVID-19 vaccine has been received by the Claiborne Medical Center (CMC) in Tazewell.
The first recipient of the vaccine is Medical Center employee Roger Mason, who manages the Emergency Department.
The first round is earmarked for those CMC employees who work in environments that are considered high risk or high exposure for COVID-19 and will be taken on a voluntary basis.
Trending
The vaccine will be administered on a planned schedule at all Covenant Health acute care facilities and will be available for home health employees and employees who work in long-term care facilities. These East Tennessee facilities include:
Claiborne Medical Center in Tazewell
Cumberland Medical Center in Crossville
Fort Loudoun Medical Center in Lenoir City
Fort Sanders Regional Medical Center in Knoxville
LeConte Medical Center in Sevierville
Trending
Methodist Medical Center in Oak Ridge
Morristown-Hamblen Healthcare System in Morristown
Parkwest Medical Center in Knoxville
Roane Medical Center in Harriman
Patti Ketterman, chief administrative officer at CMC, spoke about the vaccine and its protocol.
“Claiborne Medical Center has begun receiving our vaccine and will administer them in accordance with state, federal and CDC guidelines. These vaccines are precious and we are coordinating with all of our Covenant Health facilities to provide the vaccines to our front line staff in the most efficient way possible.
“Our team members are very excited to begin the vaccination distribution process, with our front line caregivers as the first priority. As always, our focus is on caring for our team members and our community in the best way possible,” said Ketterman.
The Pfizer COVID-19 vaccine consists of two injections administered 21 days apart. The Pfizer vaccine has been shown to be 95% effective in preventing COVID-19 seven days after the second of two doses, according to the research.
The vaccine has received extensive study under the Food and Drug Administration (FDA). The Centers for Disease Control (CDC) has guided and fully evaluated the vaccine for safety.
Rapid deployment has been made possible by producing the vaccine while being studied. This allowed quick distribution of the vaccine once safety was confirmed, using strict research protocols under the CDC and the FDA.
The FDA approves vaccines only when they are proven to be: 1) safe and effective and 2) if the benefits outweigh the risks.
Recipients may experience mild to moderate side effects – especially after the second dose – including fatigue, fever and headache. This is not a result of a COVID infection. It is the body’s response to the vaccine.
The COVID-19 vaccine is an m (messenger) RNA vaccine. It teaches the body to make antibodies to fight the COVID virus. There is no COVID virus in the vaccine, and it cannot give COVID to the vaccine recipient according to the current research.
This vaccine has specific requirements for storage and dosage. Covenant Health will follow these requirements to ensure the vaccine is administered safely, according to manufacturer and FDA standards.
Covenant Health anticipates receiving more vaccine doses in the future as they become available, and distribution will expand to other areas of the health system according to state and federal guidelines.
Following initial distribution to healthcare workers, the CDC and the FDA are coordinating plans for vaccine availability for the general public later in 2021 via physician offices, retail pharmacies and other resources.
As additional information becomes available, Covenant Health will continue to communicate with employees, patients and the communities served by the health system’s hospitals and other member organizations.
These updates are expected to include confirmation of subsequent distribution phases based on vaccine dose availability from the manufacturer, as well as direction from the FDA and CDC.
Inquiries regarding specific Covenant Health hospitals across the region may be directed to the appropriate point of contact as listed below:
Claiborne Medical Center
Betsy Maples, Manager, Marketing and Business Development
bmaples@cchnh.org
Office: 423-526-2192
Fort Sanders Regional Medical Center
Valerie Somerville, Marketing Manager
vsomervi@CovHlth.com
Office: 865-331-1542
Morristown-Hamblen Healthcare System
Peyton Moore, Marketing Manager
pmoore9@CovHlth.com
Office: 423-492-6052
Parkwest Medical Center
Amanda Paletz, Marketing Manager
apaletz@covhlth.com
Office: 865-373-1703





